The existential threat of rapid climate change to the civilization to which we have all become accustomed has prompted numerous solutions, a few of which are outright dangerous. Different geoengineering methods generally fall into two main categories: carbon dioxide (CO2) removal (CDR) and solar radiation management (SRM). Combined with reduced emissions of CO2 and other greenhouse gases (GHG), CDR aims to balance emissions and removals to achieve “Net Zero’ for climate stabilization. While CDR addresses the root cause of the problem, SRM does nothing to directly reduce the current atmospheric pool or reduce future emissions of CO2 or other GHG. This discussion focuses on SRM.
SRM includes several different approaches including cirrus cloud thinning, stratospheric aerosol injection (SAI), surface albedo geoengineering, marine cloud brightening (MCB), and the use of mirrors or sunshades in space. Cirrus cloud thinning uses various chemicals to thin clouds, theoretically increasing outgoing longwave (infrared) radiation. The other four methods will attempt to reflect the shorter, visible wavelengths of incoming sunlight back into space, reducing the amount of heat reaching the Earth’s surface and mask global warming.
Potential negative secondary effects of various proposed SRM methods have been reviewed in several recent publications1-5. Surface albedo geoengineering, which involves increasing the amount of sunlight reflected by deserts, croplands, grasslands, and urban environments, is considered to have fewer negative consequences than other approaches; however, this approach is constrained by land coverage and its potential mitigative effects are limited3. MCB attempts to inject sea salt aerosols into the marine boundary layer to make the clouds brighter and more reflective. Computer models predict the injected sea salt could reach the upper troposphere and thin the ozone later6. Additionally, the reactive chlorine and bromine species in the injected sea salts have been forecasted to increase the residence time of methane (a GHG more potent than CO2)in the atmosphere6. Others have reported MCB could alter the hydrological cycle, which would adversely affect agricultural crops7. MCB made headlines in April 2024 when large-scale experiments were conducted in San Fransico Bay8. However, to date, most research has focused on SAI.
SAI would work by injecting sulfur dioxide (SO2) into the stratosphere to create sulfate aerosols via its reaction with water vapor to reflect sunlight back into space. Because the residence time of the injected sulfur is from 0.5 to 1.3 years, a near continuous injection strategy will be required9,10. The method was first conceived in 1974 by Mikhail Budyko11 following his observations of temporary global cooling following volcanic eruptions, which naturally release huge amounts of sulfate into the stratosphere. For more information and a video that comes across like a trailer for a Sci-fi movie about aliens xenoforming Earth to suit their biology, the reader should search the internet for “The Stratoshield an Intellectual Ventures Invention”12.
One of the most often cited negative effects of SAI is ozone depletion stemming from the aerosol’s interaction with the catalytic ozone destruction cycle (by activating chlorine oxides, ClOx; bromine oxides, BrOx; and hydroxyl, HOx). This would allow more UVB to reach the Earth’s surface and increase skin cancer frequency5,13. Even with future reductions in Cl and Br (thanks to the Montreal Protocol phasing out CFCs), hydroxyl-catalyzed ozone destruction and ozone layer depletion will remain significant13.
Another potential negative impact of sulfate aerosols being injected into the stratosphere is their partial absorption of sunlight and upgoing radiation causing the stratosphere to warm. Models predict stratospheric warming could change atmospheric circulation and shift the location of the Intertropical Convergence Zone (ITCZ) altering both global and regional hydrological cycles5,14. This would likely influence the seasonal patterns and strength of monsoons and tropical storms 2,3.
As noted earlier, to illustrate the potential cooling effect of SAI, Budyko (and others) cited the cooling that followed volcanic eruptions. Negative effects have also been observed following volcanic eruptions often confirming modeling results3,5. For example, stratospheric ozone loss and changes in precipitation patterns were observed following the eruptions of both Mt. Pinatubo and El Chichón3,15. The year following Mt. Pinatubo’s eruption, land precipitation was about three standard deviations below normal16. Following low-latitude volcanic eruptions, the ITCZ was observed to shift location and the frequency of hurricanes decreased in the Atlantic17,18.
One of the chief concerns about SAI is that both its positive and negative effects will vary regionally based on injection methods, altitude, latitude, and season1,7,18. For example, injecting aerosols into only one hemisphere is forecasted to shift the ITCZ towards the other18. Models indicate that if deployed in the southern hemisphere hurricane frequency would be greater in that hemisphere and vice versa if SAI were deployed only in the northern hemisphere18. Deployment in the southern hemisphere is forecasted also to cause rain failure in northeast Brazil whereas deployment only in the northern hemisphere could cause severe droughts in sub-Saharan Africa and India7. Regional variations and unequal positive/negative impacts of SAI (only a small fraction of which are detailed above) will inevitably lead to winners and losers. This would likely raise international tensions and possibly lead to conflicts7,19, especially with unilateral SAI implementation. As highlighted in the study by the RAND Corporation7, some of the outcomes might be viewed as ‘malicious’, especially considering our past attempts to weaponize weather control20-22.
Another concern regarding SAI is that because it does nothing to address the growing CO2 problem, injections would need to increase over time with rising atmospheric CO2 and, even more alarming if it were suddenly discontinued for any reason (perhaps to ameliorate a negative secondary effect), the planet would experience “termination shock” where global temperatures would suddenly rebound to levels that it would have reached without SRM2,7,19. Such an abrupt rise in temperature could be environmentally devastating given the inability of species to adapt or migrate quickly enough.
However, the greatest risk of SRM, in my opinion, is if it is effective at masking the runaway greenhouse effect and prevents significant global warming it could potentially free decision-makers to disregard the negative effects. Should this happen, it would very likely undermine efforts to reduce GHG emissions and perhaps even fuel (pun intended) an argument for continued burning of fossil fuels23. Interestingly, the landmark 1965 report by the President’s Science Advisory Council24, which was the first government document to warn of rising CO2 and global warming, suggested the solution might be to bring about ‘countervailing climatic change’ by increasing Earth’s reflectivity but did not mention reducing GHG emissions. I don’t think it’s too much of a stretch to envision the current administration making the argument that SAI would enable us to continue to “burn, baby burn.”
If that isn’t bad enough, increased atmospheric CO2 has another, possibly even greater, environmental impact: ocean acidification (OA, Fig. 1). It may not look like a drastic drop in pH but remember this is a negative logarithmic scale (i.e., a reduction means an increase in H+ ions) so there has actually been a 15% increase in acidity since 1985. Besides the damaging impact it’s already beginning to have on marine ecosystems25, OA could weaken the ocean carbon sink thereby accelerating the runaway greenhouse effect.
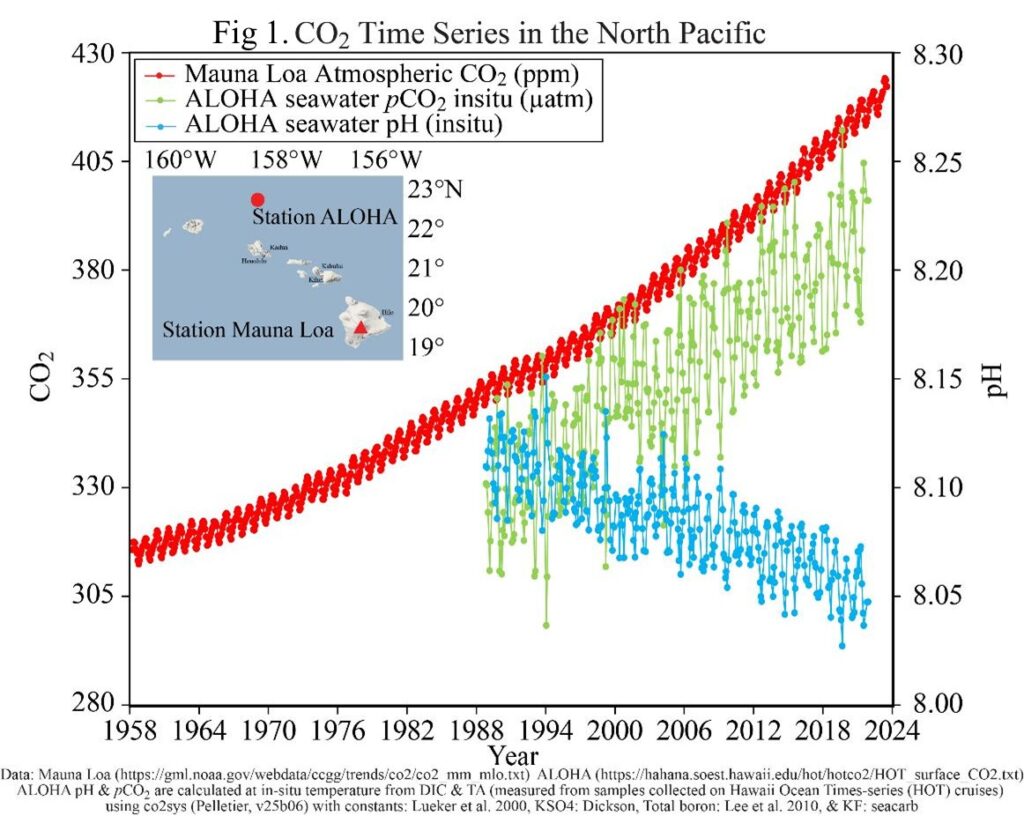
The ocean plays a vital role in the global carbon cycle, serving as a major sink or reservoir. The Global Carbon Project26 has estimated the ocean absorbed 26% of anthropogenic carbon emitted since 1850 thus serving as a negative feedback keeping the planet cooler than if all that CO2 had remained in the atmosphere. Each year from 1990 to 2015, the ocean has been estimated to sequester or remove about 2.16 gigatons of carbon (GtC), which was 45% greater than the amount removed by the terrestrial carbon sink during that time (i.e., uptake by land plants, soil)27. The Global Carbon Project26 estimated the ocean would absorb 2.9 GtC from the atmosphere in 2023; equal to that of the terrestrial sink.
Regrettably, in any discussion of ocean acidification and its potential impact on air-sea carbon exchange, there’s just no getting around the chemistry, which can be quite complex (confused readers are directed to the wealth of online educational materials 28,29). For the purposes of this discussion, it is important for the reader to understand that unlike other atmospheric gases (e.g., N2 and O2), CO2 chemically reacts with water when dissolved to form the carbonate system (Fig. 2) that can buffer the ocean’s pH by giving off a hydrogen ion (under less acidic conditions and thereby increasing acidity) or by taking up a hydrogen ion (under more acidic conditions and thereby reducing pH).
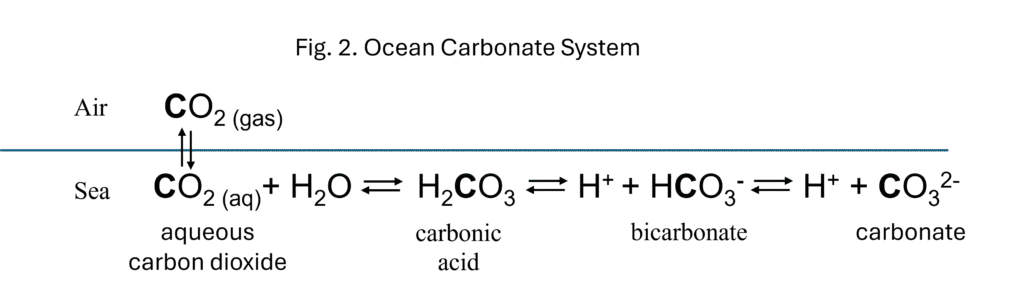
These different carbonate species also play vital roles in three different but interlinked processes controlling carbon transport and flux in or out of the ocean. These processes are termed carbon pumps because they can transfer carbon from the atmosphere to the deep ocean where it remains on timescales of several centuries to millennia before returning to the surface30.
The first process known as the ‘solution (or solubility) pump’ is an abiotic process involving the influx, efflux and transport of dissolved inorganic carbon (DIC, i.e., the sum of the inorganic forms: CO2, H2CO3, HCO3–, CO32-) throughout the water column.
The second process is the ‘soft tissue (or organic) pump’ where phytoplankton produces tissues consisting of organic matter thus removing some of the DIC (including CO2 and HCO3–) from surface waters. These tissues, and tissues of animals that feed on these plants, eventually sink. However, as these dead organisms and fecal matter sink, they may be decomposed by microbial action at different depths releasing DIC back into the water that can be transported back to the surface (note, the sinking organic matter can under certain conditions be buried under sediment and after a time transformed into fossil fuels, e.g., oil, gas and methane hydrates for long-term storage of carbon).
The third process is the so-called ‘hard tissue (or carbonate) pump’ consisting of the incorporation of dissolved chemicals (CO32- and calcium) into shells of CaCO3 by marine organisms known as calcifiers that ultimately sink and are buried or dissolve at depth. Note, paradoxically, in utilizing two HCO3– to produce a single CaCO3, biogenic calcite production actually generates a CO2, unless it is used in photosynthesis by phytoplankton31. So, while shell deposits like the White Cliffs of Dover can be a long-term sink, their production can be a short-term source of CO2.
Another piece of fundamental chemistry you must understand is that while thermodynamic potential (and in this case solubilities) will determine equilibrium concentrations of reactants and products, the chemical reaction and the final concentrations will shift in one direction or the other due to changes in pressure, temperature, or concentration of the reactants or products. This is known as Le Châtelier’s Principle and is heavily utilized in the chemical industry. So, for example, as the dissolved gaseous CO2 chemically reacts to form carbonic acid, its concentration in the water is effectively reduced and therefore the equilibrium is shifted so that additional gaseous CO2 can dissolve. This is why the total amount of CO2 is so much greater in the ocean than the other gases like N2 and O2 (which are at much higher concentration in the atmosphere but stay in the gaseous form when dissolved). The rate of conversion of CO2 into the carbonate species is measured as the Revelle factor32 and is dependent on the pH and buffering capacity of the ocean. As another example of Le Châtelier’s Principle, as CO2 concentration increased in the atmosphere following the Industrial Revolution, the equilibrium was shifted and the rate of influx into the ocean increased. The equilibrium is also shifted when dissolved concentrations of CO2 or HCO3– are reduced in surface waters when taken up for photosynthesis to make tissues which allows for more CO2 to dissolve in the ocean. Alternatively, if dissolved concentrations are increased in surface waters relative to atmospheric partial pressure or temperature-dependent solubilities, for example in areas of upwelling of deep water rich in DIC from decomposed tissues, efflux or outgassing of CO2 into the atmosphere will be favored.
With this background, we can now discuss the conceptual impacts (no modeling was done for this analysis) of ocean acidification on the ocean sink. The scenario we are going to assess presupposes that coordinated implementation of SAI has effectively prevented global warming but there have been little to no efforts to control GHG emissions. It is important to remember the conditions of the counterfactual because several papers report on the effect global warming may have on the ocean sink, i.e., from increased sea surface temperatures, changes in temperature-dependent solubility, wind velocity, stratification, ocean currents and productivity, with most reporting an increase in net primary production, particularly in the Southern Ocean, and export of carbon by the soft tissue pump33. However, because the effect of warming on the solution pump and soft tissue pump often differs in direction due to increased CO2 outgassing, they tend to offset each other thus diminishing the overall impact warming alone has on the ocean carbon sink34-36. And remember, under this scenario, SAI is preventing global warming and any potential positive effects it may have on primary production.
Fewer studies have modeled and forecasted OA’s impact on the ocean carbon sink with almost none separating its impact from that of global warmingcf. 37. It is clear, however, that OA has the potential to significantly alter many of these processes but the magnitude and direction of these changes in regard to their impact on the ocean carbon sink remains highly uncertainty38. At lower pH the ocean’s buffering capacity should be reduced (due to lower alkalinity)altering the Revelle factor which should lessen the conversion rate and influx of gaseous CO239. If this were the case, it would serve as an amplifying feedback worsening the CO2 problem in the atmosphere. However, a pH-dependent response observed in two of the ocean’s major calcifiers, the foraminifera and coccolithophores, may mitigate this “buffer erosion” to some extent. When cultured in the laboratory under more acidic conditions, foraminifera have been found to reduce shell calcification40. Modeling of coccolithophores under high CO2 suggests they also would reduce shell calcification41. Consequently, the calcifiers would use less bicarbonate (HCO3–) thus reducing their contribution to the alkalinity erosion of surface waters40. This increased alkalinity (or more appropriately viewed as less of an expected decrease absent this effect) would enhance CO2 uptake by the oceans via the solution pump40,42. However, reduced calcification also means reduced density (i.e. less blasting of the shell) that could reduce gravitational sinking and reduce soft tissue pumping. This is thought to be the case for coccolithophores41. Yet, the sinking rate of the foraminifera was actually found to increase due to lower production of calcite spines, which when present tend to slow the descent of the forams, so their export of soft tissue to depth would be continued, if not increased40.
While OA research focuses mostly on calcifiers, its effects extend to non-calcifying phytoplankton such as diatoms, which can also influence the carbon cycle despite their silica shells (i.e., via the soft tissue pump). Taucher et al.43 found diatom shells dissolve more slowly when cultured under more acidic conditions resulting in increased export of solid silica to deeper waters. In a warmer world, this slower dissolution might be offset by faster dissolving of silica at higher temperatures; however, remember in this scenario SAI has mitigated global warming. Increased export of silica from surface water reduces the availability of regenerated silicic acid, for the production of new shells. Simulations by Taucher et al.43 suggest this could result in a 13% to 26% decline in diatoms across the world’s oceans which would be significant because diatoms typically account for up to 40% of all marine primary production and particulate organic carbon exported to depth. On a side note, these findings may give pause to those advocating for iron fertilization of the ocean as a CDR method, as they hoped to stimulate diatom blooms to drawdown CO2, not calcifying organisms, (i.e., to avoid erosion of buffering capacity)44. Still, another group of important non-calcifying primary producers, possibly the dominant primary producers in the open ocean, for which almost nothing is known in terms of their OA vulnerability, are the cyanobacterial picoplankton. One study in which a strain of Synechococcus, one of the main groups of marine cyanobacteria, was cultured under different pH conditions, found its growth negatively affected at lower pH 45. Equally important, the study also found lower pH affected the relationship between this picoplankton and the virus (i.e., cyanophage) that often infects them. Specifically, in negatively affecting the physiological state of the host, infection rate, cyanophage production and release of progeny were reduced45. While picoplankton are thought to be the most abundant photosynthetic organisms in the oceans, viruses are the most abundant biological entities in the oceans46 and through viral-induced host mortality, lysis, and the viral shunt of released organic material to the microbial loop (i.e., rather than zooplankton eating phytoplankton, organic material is decomposed by bacteria) play critical roles in the biological pumps46. Microbiologists from 35 institutions from around the world warned in a 2019 consensus statement that climate change effects will depend heavily on microbial responses, notably from understudied marine cyanobacteria and viruses47.
Recent events may foreshadow future consequences if the ocean sink is disrupted. Between 2022 and 2024, the rate at which CO2 increases in the atmosphere spiked at levels not previously observed in the 56 years of atmospheric monitoring (i.e., Keeling curve data,48). Not coincidentally, air and ocean temperatures soared in 2023 and 2024, breaking many records49,50. Those increased temperatures are thought to have resulted in increased intensity of heat waves (leading to deaths), extreme storms, crop failures, and wildfires51. What is particularly alarming about this is that the cause of this spike in atmospheric CO2 was not believed to be a sudden increase in CO2 emissions but instead likely resulted from a weakening of the terrestrial carbon sink due to drought in the Amazon and wildfires in Canada and Southeast Asia52,53. Hopefully, this is just temporary, and the terrestrial carbon sink will rebound with sufficient rain. Picture a future where increasing ocean acidification weakens the ocean carbon sink, perhaps permanently.
In conclusion, the potential for unintended, harmful effects from using solar geoengineering to mask the runaway greenhouse effect and prevent further warming (only while it’s being implemented) is significant. SRM therefore requires much more research and should be viewed with extreme caution. In my opinion, the only acceptable reason for using SRM, given its failure to address ocean acidification, is if significant headway is made on emission cuts and CDR, but additional time is required to achieve Net Zero and avert sudden catastrophe. We should therefore immediately redouble our efforts to address the root cause of the problem. You decide and be sure to voice your informed opinion when someone in power suggests implementing SAI, particularly if they propose to do it unilaterally without first implementing CDR methods and reductions in GHG emissions.
References
7. Grisé M, Yonekura E, DeSmet D, Garg A, Blake JS, Preston BL. Climate Control: International Legal Mechanisms for Managing the Geopolitical Risks of Geoengineering. RAND Corporation 2021. Available online: https://www.rand.org/pubs/perspectives/ PEA1133-1.html.
11. Budyko MI. On present‐day climatic changes. Tellus. 1977;29(3):193-204.
12. The Stratoshield an Intellectual Ventures Invention. https://www.youtube.com/ watch?v=JrimZzgqwdo
14. MacMartin DG, Ricke KL, Keith DW. Solar geoengineering as part of an overall strategy for meeting the 1.5 °C Paris target. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2018;376(2119):20160454.
15. National Research Council (NRC). Climate intervention: Reflecting sunlight to cool Earth. National Academies Press; 2015. Available online: https://nap.nationalacademies.org/catalog /18988/climate-intervention-reflecting-sunlight-to-cool-earth
21. Fleming JR. The climate engineers. The Wilson Quarterly (1976-). 2007;31(2):46-60.
24. Revelle R, Broecker W, Craig H, Keeling CD, Smagorinsky J. Appendix Y4: Atmospheric carbon dioxide.ed: In: Hornig J, York HF, Branscomb LM et al.(eds,) President’s Science Advisory Committee. Restoring the Quality of Our Environment. Washington: The White House, 1965, 111–33
28. https://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification.
30. Broecker WS, Peng TH. Tracers in the Sea. New York Eldigio Press; 1982.
44. Smetacek V, Naqvi S. The next generation of iron fertilization experiments in the Southern Ocean. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2008;366(1882):3947-67.
45. Traving SJ, Clokie MR, Middelboe M. Increased acidification has a profound effect on the interactions between the cyanobacterium Synechococcus sp. WH7803 and its viruses. FEMS microbiology ecology. 2014;87(1):133-41.
47. Cavicchioli R, Ripple WJ, Timmis KN, Azam F, Bakken LR, Baylis M, et al. Scientists’ warning to humanity: microorganisms and climate change. Nature Reviews Microbiology. 2019;17(9):569-86.
52. Ke P, Ciais P, Sitch S, Li W, Bastos A, Liu Z, et al. Low latency carbon budget analysis reveals a large decline of the land carbon sink in 2023. National Science Review. 2024;11(12):nwae367.
